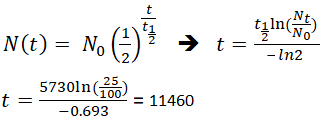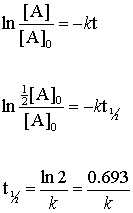half life formula chemistry
In order to calculate the half-life of a chemical specie integrated half-life equations are used according to the order of reactions. All 10 grams were carbon.

First Order Reaction Definition Example Half Life Period Chemistry Notes
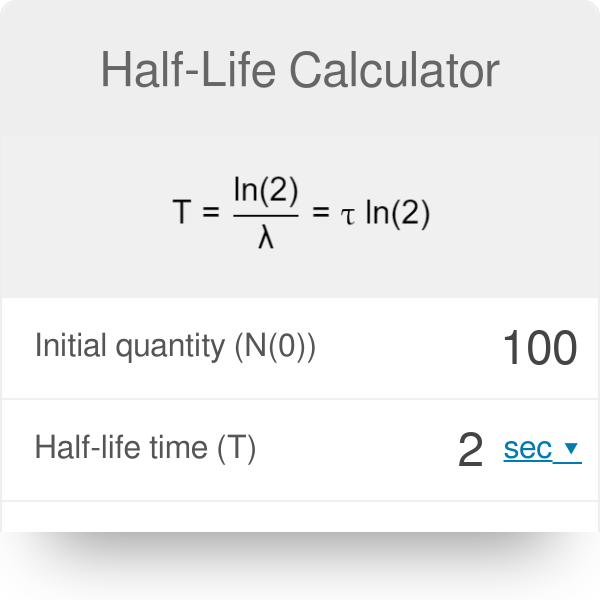
The formula for a half-life is T12 ln2 λ.

. T is the half-life. For a zero-order reaction the half-life equation is given as. The half-life of a first-order reaction does not depend upon the.
Half-life Half-life thalf is defined as the amount of time required for the amount of a substance to be reduced by 50. So we started with this. This chemistry video tutorial shows explains how to solve common half life radioactive decay problems.
So now you have after one half-life-- So lets ignore this. The formula for half-life in chemistry depends on the order of. Substitute this information into the equation for the half life of a reaction with this order and solve for t ½.
Given half life of the substance is t1 2 t 1 2 004. For a first zero order. Where t 12 is the half life and k is the rate.
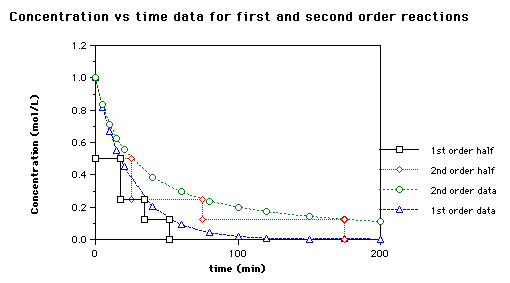
Kinetics we can determine the half-life formula is. Half-life symbol t 12 is the time required for a quantity to reduce to half of its initial valueThe term is commonly used in nuclear physics to describe how quickly unstable atoms undergo. The half-life of a reaction is the time required for the reactant concentration to decrease to one-half its initial value.
N t 1 2 n X N o 1 2 4 X 50 3125 g. It shows you a simple technique to find the final amo. The equations are given above.
We utilize the equation that relate amount remaining initial mass and number of half-livesn. So if you go back after a half-life half of the atoms will now be nitrogen. Half life is based around the decay process and the number of unstable nuclei remaining after time t.
The half-life formula for various reactions is given below. In nuclear reactions this time period. For a zero order reaction A products rate k.
Half-life a useful concept if its value does not depend on how much. The mathematical expression that can be employed to determine the half-life for a zero-order reaction is t12 R 02k. Converting a Half Life to a Rate Constant.
T 12 ln2k. We know that at the half-life time eqt_12 eq the concentration of the reactant will. Find the value of the decay constant of a radioactive substance having a half-life of 004 seconds.
Mean half life calculator uses the half life formula to compute results. By applying the first order integrated rate law to this described in Chapter 12. Using the concentration-time equation for a second-order reaction we can solve for half-life.
The half-life formula for a reaction depends upon the order of a reaction. For example if the half-life of a. A radioactive half-life refers to the amount of time it takes for half of the original isotope to decay.
C-14 Half-Life 5730 Years.

Nuclear Chemistry Half Life Transmutation 6 Practice Problems With Answer Key

Half Life Expressions Chemistnate

Nuclear Half Life Calculations Youtube

Half Life Calculator Radioactive Decay Calculator

C 3 Calculating The Decay Constant Sl Youtube
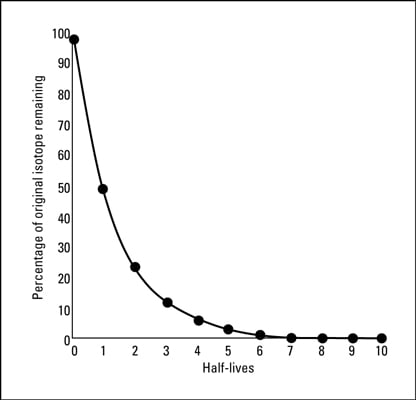
Nuclear Chemistry Half Lives And Radioactive Dating Dummies
Half Life Introductory Chemistry

Radioactive Decay Formula Radioactive Half Life 0 693 Radioactive Decay Constant Physics Topics Science Themes Physics
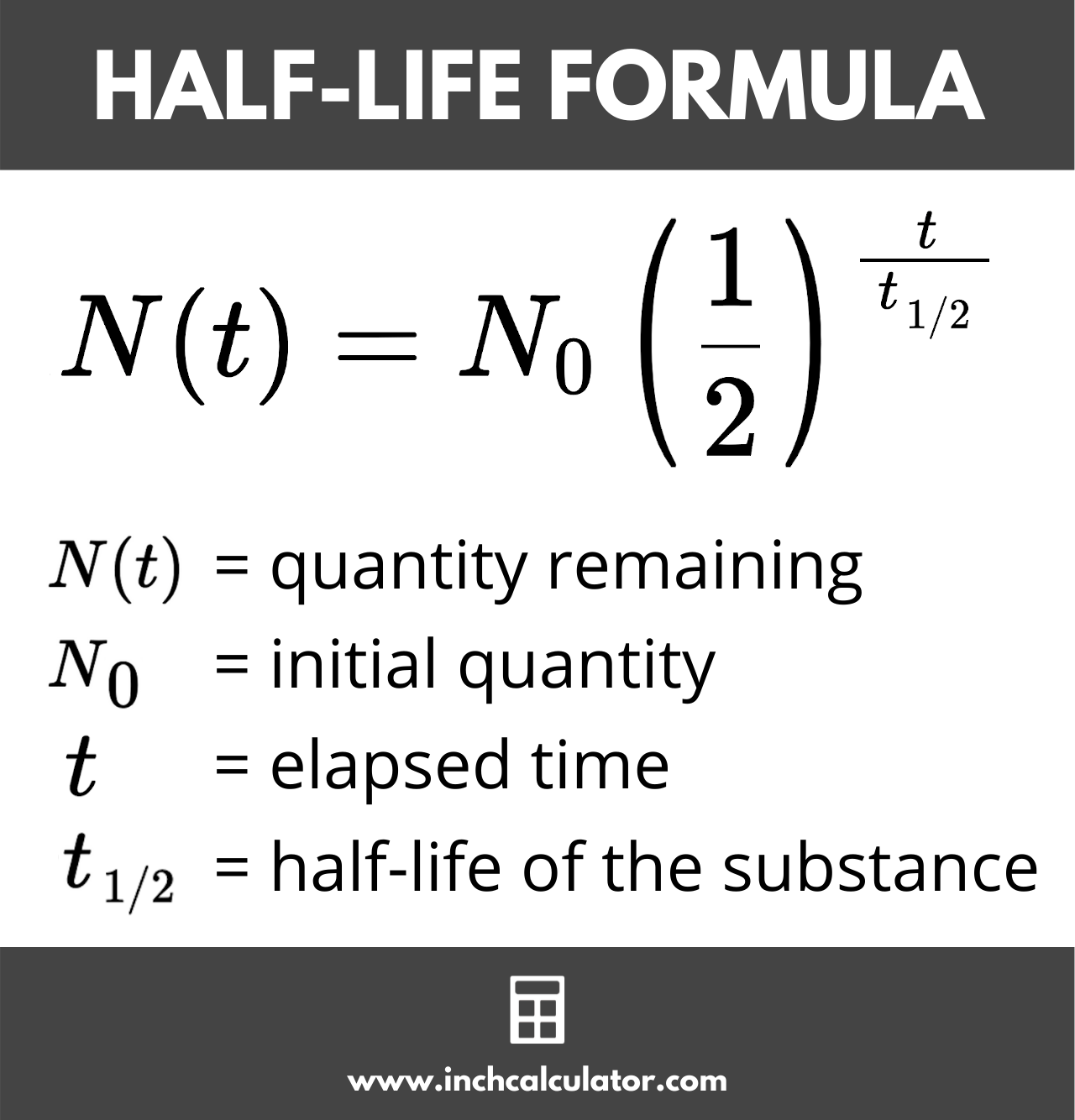
Half Life Calculator Inch Calculator
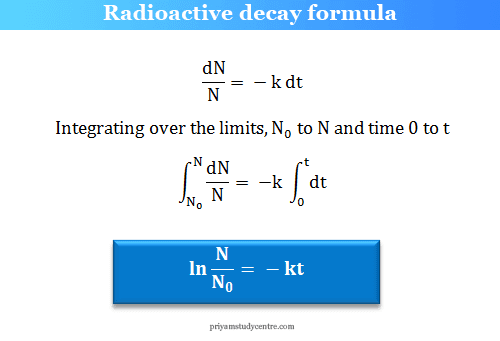
Radioactive Decay Half Life Definition Formula Calculation

Identifying Half Life Given The Rate Constant Chemistry Study Com